
FDA Grants Accelerated Approval to Otsuka’s VOYXACT for IgAN Proteinuria Reduction
Otsuka Announces FDA Accelerated Approval of VOYXACT® for Proteinuria Reduction in Adults with Primary IgA Nephropathy Otsuka Pharmaceutical Development & Commercialization, Inc., together with Otsuka Pharmaceutical Co., Ltd., announced that…

Arrowhead Pharmaceuticals Announces Full-Year 2025 Financial Results
Arrowhead Pharmaceuticals Reports Fiscal Year 2025 Financial Results and Highlights Major Regulatory and Clinical Milestones Arrowhead Pharmaceuticals, Inc. (NASDAQ: ARWR) today announced its financial results for the fiscal year ending…
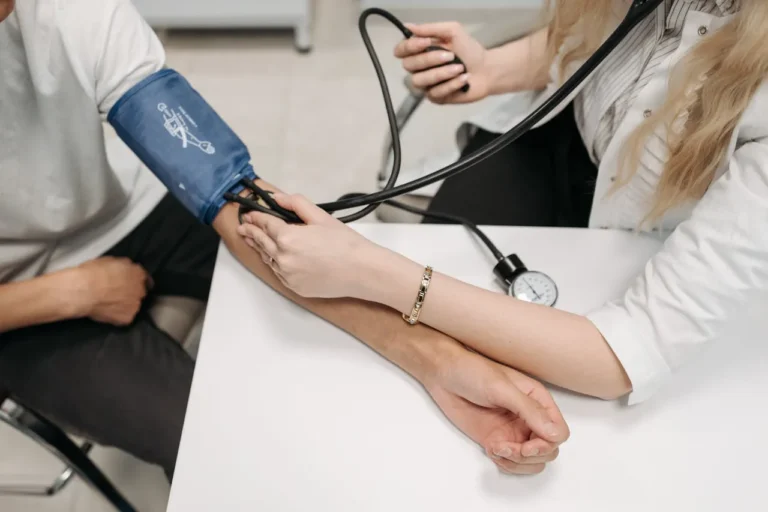
Real-World Evidence Confirms Repatha’s Sustained Cardiovascular Benefits
Real-World Study Shows Durable Cardiovascular Protection with Repatha Target RWE, a leading organization in modern real-world evidence (RWE) generation and a trusted partner for regulatory-grade research, has announced significant new…

EdgeOne Medical Names Marty Shelton Chief Operating Officer
EdgeOne Medical Appoints Industry Veteran Marty Shelton as Chief Operating Officer to Advance Operational Excellence, Client Value, and Long-Term Growth EdgeOne Medical, a trusted provider of development and testing services…
Allurion Reports Early Results Combining Program With Low-Dose Tirzepatide
Allurion Unveils Initial Results on Program + Tirzepatide Combo Allurion Technologies, Inc. (“Allurion” or the “Company”) (NYSE: ALUR)—a global leader in innovative, non-surgical weight-loss technology—today announced encouraging initial results from…

Longhorn Validates PrimeStore® MTM for Global TB Screening
PrimeStore® MTM Shows Strong Validation for Global TB Testing Longhorn Vaccines & Diagnostics (“Longhorn”), a One Health-focused biotechnology company advancing innovative diagnostics and antibody platforms to address global public health…

ReAlta Life Sciences Announces Appointment of Dr. Howard Berman as Executive Chairman
ReAlta Life Sciences Strengthens Leadership Team with Appointment of Dr. Howard Berman as Executive Chairman ReAlta Life Sciences Inc. (“ReAlta” or the “Company”), a clinical-stage biopharmaceutical company pioneering next-generation medicines…
Zetagen Therapeutics Raises Oversubscribed Series B1 Round
Zetagen Therapeutics Announces Oversubscribed $12.9M Series B1 Financing to Advance Breakthrough Intratumoral Therapies for Breast Cancer Zetagen Therapeutics, Inc., a privately held clinical-stage biopharmaceutical company advancing first-in-class intratumoral therapies for…

Enigma Biomedical USA Receives MJFF Grant for Novel α-Synuclein PET Biomarker
Enigma Biomedical USA Awarded $2 Million Grant from The Michael J. Fox Foundation to Advance Novel α-Synuclein PET Imaging Biomarker for Parkinson’s Disease Enigma Biomedical USA (EB USA) today announced…

Vyome Appoints Dr. Aditya Bardia as Senior Medical Advisor
Vyome Holdings Announces Appointment of Renowned Oncologist Dr. Aditya Bardia as Senior Medical Advisor to Advance VT-1953 for Malignant Fungating Wounds Vyome Holdings, Inc. (“Vyome”) (NASDAQ: HIND), a clinical-stage healthcare…

Revised Announcement: Pathway to Cures Invests in SeraGene Therapeutics
Pathway to Cures Invests in SeraGene Therapeutics The fifth paragraph of the original release dated November 19, 2025, should read: Pathway to Cures joins Hextwo Capital as early investors in…

SHORE Study Confirms HeartCare’s Prognostic Power in Transplant Patients
CareDx Announces Publication of New SHORE Study Demonstrating the Prognostic Power of HeartCare® for Heart Transplant Recipients in the Journal of Heart and Lung Transplantation CareDx, Inc. (Nasdaq: CDNA), a…


