
FDA Approves AKEEGA®—First Precision Therapy for BRCA2+ mCSPC, Cutting Progression Risk by 54%
U.S. FDA Approves AKEEGA®—First Precision Therapy for BRCA2-Mutated Metastatic Castration-Sensitive Prostate Cancer, Cutting Disease Progression by 54% Compared to Standard of Care* Johnson & Johnson announced today the U.S. Food…

EMA Recommends Higher Wegovy® Dose for 20.7% Average Weight Loss in Obesity
Novo Nordisk A/S: EMA Recommends Higher-Dose Wegovy® for Obesity, Delivering Average Weight Loss of 20.7% Today, the European Medicines Agency’s (EMA) Committee for Medicinal Products for Human Use (CHMP) issued…

Updated Data Reinforce Inluriyo™ (imlunestrant) Efficacy—Monotherapy and with Verzenio®—in ER+/HER2− Advanced Breast Cancer
Updated Data for Lilly’s Inluriyo™ (imlunestrant) Reinforce Efficacy as Monotherapy and in Combination with Verzenio® (abemaciclib) in ER+, HER2– Advanced Breast Cancer Eli Lilly and Company today announced updated results…

Sarepta Therapeutics Completes Refinancing of $291 Million in 1.25% Convertible Senior Notes Due 2027
Sarepta Therapeutics Completes Refinancing of $291 Million in 1.25% Convertible Senior Notes Due 2027 Sarepta Therapeutics, Inc., the leader in precision genetic medicine for rare diseases, today announced that it…

Bio-Techne and Wyss Center Geneva Collaborate to Advance Automated 3D Multiomics and Accelerate Spatial Biology
Bio-Techne Collaborates with Wyss Center Geneva to Drive Innovation in Automated 3D Multiomics and Accelerate Spatial Biology Research Bio-Techne Corporation, a global provider of life science tools, reagents, and diagnostic…

Alnylam Announces Partial Buyback of 1.00% Convertible Senior Notes Due 2027
Alnylam Announces Partial Buyback of 1.00% Convertible Senior Notes Maturing in 2027 Alnylam Pharmaceuticals, the leading RNAi therapeutics company, announced today that it has entered into separate, privately negotiated repurchase…

FDA Approves Blujepa (Gepotidacin) as First Oral Treatment for Uncomplicated Urogenital Gonorrhea
Blujepa (gepotidacin) Receives US FDA Approval as Oral Treatment for Uncomplicated Urogenital Gonorrhea (uGC) GSK plc today announced that the US Food and Drug Administration (FDA) has approved a supplemental…
FDA Grants Priority Review to Bristol Myers Squibb’s Opdivo® Plus Chemo for Hodgkin Lymphoma
FDA Grants Priority Review to Bristol Myers Squibb’s Opdivo® (nivolumab) Plus Chemotherapy for Classical Hodgkin Lymphoma Bristol Myers Squibb today announced that the U.S. Food and Drug Administration (FDA) has…
Roche Expands Mass Spec Menu with CE-Marked Antibiotic Monitoring—Broadest IVD Offering
Roche Expands Automated Mass Spectrometry Portfolio with CE Mark Approval for Antibiotic Drug Monitoring, Delivering the Industry’s Broadest IVD Menu Roche announced today that it has secured CE Mark approval…
FDA Approves Uplizna® for the Treatment of Generalized Myasthenia Gravis in Adults
FDA Approves UPLIZNA® for Use in Adults with Generalized Myasthenia Gravis Amgen today announced that the U.S. Food and Drug Administration (FDA) has approved UPLIZNA® (inebilizumab-cdon) for the treatment of generalized myasthenia gravis…

TUKYSA Boosts PFS by 8+ Months in First-Line HER2+ Metastatic Breast Cancer
TUKYSA Added to First-Line Maintenance Therapy Boosts Median Progression-Free Survival by More Than 8 Months in HER2+ Metastatic Breast Cancer Patients Pfizer Inc. today announced detailed results from the Phase…
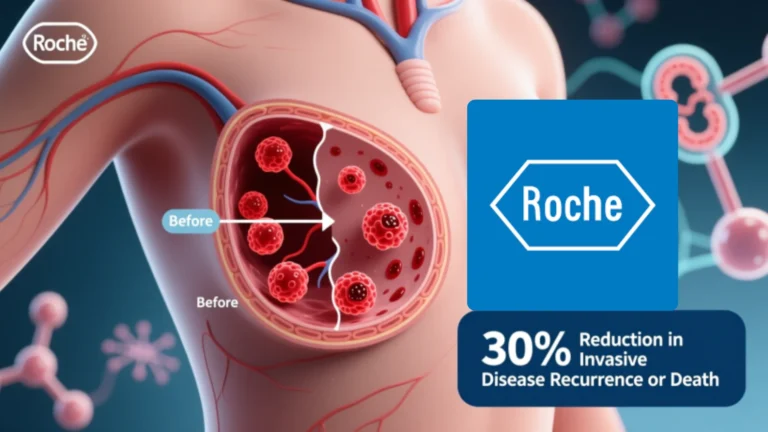
Roche’s Giredestrant Cuts Risk of Invasive Disease Recurrence or Death by 30% in ER-Positive Early-Stage Breast Cancer
Roche’s Giredestrant Lowers Risk of Invasive Disease Recurrence or Death by 30% in Early-Stage ER-Positive Breast Cancer Roche announced today positive data from the phase III lidERA Breast Cancer study…


