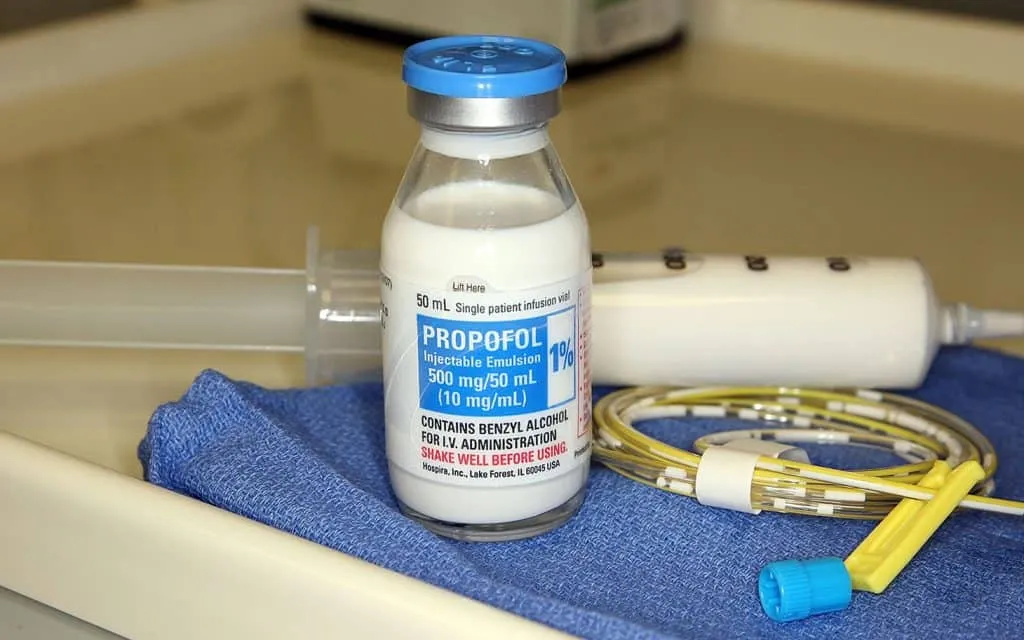
Avenacy Introduces Propofol Injectable Emulsion, USP to the U.S. Market
Avenacy Launches FDA-Approved Propofol Injectable Emulsion, USP in the United States: A Milestone in Critical Care and Anesthesia Access
Avenacy, a rising force in the specialty pharmaceutical sector, announced today the official U.S. launch of Propofol Injectable Emulsion, USP, a therapeutic generic equivalent to the widely used anesthetic Diprivan® (propofol). Approved by the U.S. Food and Drug Administration (FDA), Avenacy’s version of propofol will now be available nationwide and represents a significant stride in enhancing access to critical care injectable medications across hospital and clinical settings.
This move reflects Avenacy’s continued commitment to bolstering the availability of essential generic medications for high-acuity environments, such as surgical centers, intensive care units, and emergency departments. With the introduction of Propofol Injectable Emulsion, USP, the company not only expands its growing portfolio of FDA-approved injectables but also aims to address persistent challenges in the supply and reliability of critical anesthetic agents.
A Closer Look at Propofol Injectable Emulsion, USP
Propofol is a short-acting intravenous general anesthetic agent that plays a central role in modern anesthesia and sedation protocols. Since its initial approval in the 1980s, propofol has become a mainstay in operating rooms, procedural sedation, and critical care settings worldwide.
Avenacy’s Propofol Injectable Emulsion, USP, mirrors the reference listed drug Diprivan® in formulation, pharmacokinetics, and therapeutic use. The product has received FDA approval as a generic equivalent, meaning it meets rigorous standards for safety, efficacy, and quality.
According to the company’s announcement, the newly launched product is indicated for multiple clinical applications across different patient populations. These include:
- Induction of General Anesthesia for patients aged 3 years and older
- Maintenance of General Anesthesia for patients aged 2 months and older
- Initiation and Maintenance of Monitored Anesthesia Care (MAC) Sedation in adult patients
- Sedation in Combination with Regional Anesthesia for adult patients
- Sedation in Mechanically Ventilated Adult Patients in the Intensive Care Unit (ICU)
These indications reflect propofol’s versatility in clinical practice—from outpatient procedures and surgeries to long-term sedation in critical care units.
Packaging Innovation and Safety Measures
In line with its mission to champion patient safety and improve workflow efficiency for healthcare providers, Avenacy has designed its propofol product with highly distinctive, user-friendly packaging. The goal is to reduce the risk of medication errors, a common concern in fast-paced hospital environments where multiple injectable medications are used concurrently.
Propofol Injectable Emulsion, USP will be offered in three different vial sizes to accommodate a range of dosing needs:
- 200 mg/20 mL single-dose vial
- 500 mg/50 mL single-dose vial
- 1,000 mg/100 mL single-dose vial
The single-dose format minimizes contamination risks and supports best practices in infection control. Each vial is clearly labeled with differentiated design elements to facilitate accurate selection during time-sensitive procedures.
“Ensuring that essential medications like propofol are available in dependable, clearly labeled formats is crucial to supporting front-line healthcare workers and improving outcomes for patients in need of sedation or anesthesia,” said a spokesperson from Avenacy. “Our packaging innovations are aligned with the growing emphasis on patient safety and medication accuracy across U.S. healthcare systems.”
Strategic Manufacturing and Supply Chain Reliability
Avenacy has emphasized its strong manufacturing and development infrastructure in support of this launch. The company has forged strategic relationships with a global network of contract manufacturing organizations (CMOs) and pharmaceutical development partners, all of whom operate in compliance with current Good Manufacturing Practices (cGMP). These partners have successfully passed FDA inspections, underscoring Avenacy’s commitment to quality, reliability, and regulatory compliance.
Shipments of Propofol Injectable Emulsion, USP are expected to begin immediately, with wholesale partners already slated to receive initial batches within the week. By partnering with wholesale distribution networks, Avenacy aims to ensure the drug reaches hospitals, surgical centers, and critical care facilities across the country with minimal delay.
The market for propofol in the United States is substantial. For the 12-month period ending December 2024, total U.S. sales of propofol injectable emulsion reached an estimated $315 million, highlighting the drug’s essential role in modern medical care and the strong demand for quality-assured generics.
Pediatric Limitations and FDA Guidance
While propofol is approved for several adult and pediatric anesthesia applications, the FDA has outlined specific limitations regarding its use in younger pediatric populations. As such, Avenacy’s Propofol Injectable Emulsion, USP includes the following limitations of use per FDA labeling guidelines:
- Not recommended for the induction of anesthesia in patients under 3 years of age, as safety and efficacy data are lacking for this group.
- Not indicated for maintenance of general anesthesia in patients under 2 months of age due to insufficient clinical data.
- Not approved for use in pediatric patients undergoing Monitored Anesthesia Care (MAC) sedation, as the safety and effectiveness of propofol for this use remain unestablished.
- Not indicated for Pediatric ICU sedation, as there is no established safety profile for long-term propofol sedation in critically ill children.
These restrictions reflect a cautious, evidence-based approach to pediatric dosing, and clinicians are advised to refer to full prescribing information and FDA guidance when treating patients in these age groups.
Enhancing Access to Critical Injectable Medications
With today’s launch, Avenacy reinforces its position as a specialty pharmaceutical company focused on addressing key gaps in the U.S. injectable drug market. The company has made it a core mission to expand the availability of high-priority medications, particularly those used in hospitals and acute care settings.
The U.S. healthcare system has experienced recurring shortages of injectable anesthetics and sedatives over the years, often due to manufacturing disruptions, supply chain vulnerabilities, and quality control issues. By introducing a high-quality generic alternative to Diprivan® that is backed by FDA approval and robust manufacturing processes, Avenacy aims to bring greater stability and resilience to the propofol supply chain.
This is not Avenacy’s first step into the critical care therapeutic space. The company has been steadily growing its portfolio of sterile injectables, targeting areas with documented unmet needs and limited supplier diversity. Future plans include the launch of additional anesthetic, analgesic, and critical care formulations over the coming quarters.
A Step Forward in Anesthesia Access and Safety
The availability of Avenacy’s Propofol Injectable Emulsion, USP in the United States marks a significant milestone not just for the company but for healthcare providers and patients alike. With FDA-approved therapeutic equivalence to Diprivan®, distinctive packaging that supports medication safety, and a proven global manufacturing backbone, this launch is expected to make a meaningful impact in the delivery of anesthesia and sedation across U.S. hospitals.
As Avenacy continues to expand its presence in the pharmaceutical landscape, its emphasis on quality, access, and innovation positions it as a valuable contributor to the future of critical care and specialty drug delivery.
For more detailed prescribing information, safety warnings, and administration guidelines, healthcare professionals are encouraged to consult the full Prescribing Information approved by the FDA.





