
Alnylam Announces Partial Buyback of 1.00% Convertible Senior Notes Due 2027
Alnylam Announces Partial Buyback of 1.00% Convertible Senior Notes Maturing in 2027 Alnylam Pharmaceuticals, the leading RNAi therapeutics company, announced today that it has entered into separate, privately negotiated repurchase…

FDA Approves Blujepa (Gepotidacin) as First Oral Treatment for Uncomplicated Urogenital Gonorrhea
Blujepa (gepotidacin) Receives US FDA Approval as Oral Treatment for Uncomplicated Urogenital Gonorrhea (uGC) GSK plc today announced that the US Food and Drug Administration (FDA) has approved a supplemental…
FDA Grants Priority Review to Bristol Myers Squibb’s Opdivo® Plus Chemo for Hodgkin Lymphoma
FDA Grants Priority Review to Bristol Myers Squibb’s Opdivo® (nivolumab) Plus Chemotherapy for Classical Hodgkin Lymphoma Bristol Myers Squibb today announced that the U.S. Food and Drug Administration (FDA) has…
Roche Expands Mass Spec Menu with CE-Marked Antibiotic Monitoring—Broadest IVD Offering
Roche Expands Automated Mass Spectrometry Portfolio with CE Mark Approval for Antibiotic Drug Monitoring, Delivering the Industry’s Broadest IVD Menu Roche announced today that it has secured CE Mark approval…
FDA Approves Uplizna® for the Treatment of Generalized Myasthenia Gravis in Adults
FDA Approves UPLIZNA® for Use in Adults with Generalized Myasthenia Gravis Amgen today announced that the U.S. Food and Drug Administration (FDA) has approved UPLIZNA® (inebilizumab-cdon) for the treatment of generalized myasthenia gravis…

TUKYSA Boosts PFS by 8+ Months in First-Line HER2+ Metastatic Breast Cancer
TUKYSA Added to First-Line Maintenance Therapy Boosts Median Progression-Free Survival by More Than 8 Months in HER2+ Metastatic Breast Cancer Patients Pfizer Inc. today announced detailed results from the Phase…
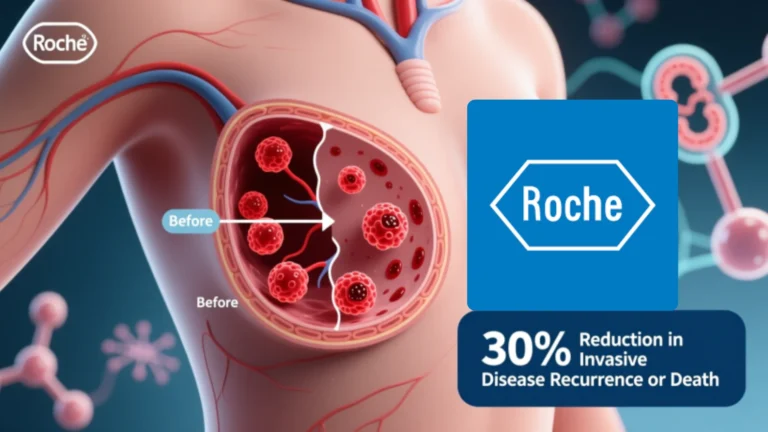
Roche’s Giredestrant Cuts Risk of Invasive Disease Recurrence or Death by 30% in ER-Positive Early-Stage Breast Cancer
Roche’s Giredestrant Lowers Risk of Invasive Disease Recurrence or Death by 30% in Early-Stage ER-Positive Breast Cancer Roche announced today positive data from the phase III lidERA Breast Cancer study…
Teva Submits NDA for Once-Monthly Olanzapine Injection for Schizophrenia
Teva Pharmaceuticals Files New Drug Application with FDA for Once-Monthly Olanzapine Extended-Release Injectable Suspension (TEV-‘749) to Treat Schizophrenia in Adults Teva Pharmaceuticals, a U.S. affiliate of Teva Pharmaceutical Industries Ltd.,…
ICON 2025 Survey Shows China Leading Biotech, Highlights Opportunities for Western Firms
ICON plc, a world-leading clinical research organisation, today released findings from two biotech sector surveys. The first survey focused on global biotech trends and is an update of our 2023…

AskBio’s AB-1005 and AB-1002 Granted Japan’s Pioneering Regenerative Medicine Product Designation
AskBio’s AB-1005 and AB-1002 Granted Pioneering Regenerative Medicine Product Designation in Japan AskBio Inc., a gene therapy company wholly owned and independently operated as a subsidiary of Bayer AG, today…

CVS Health Revises Guidance, Showcases Business Strength, and Unveils Healthcare Reimagining Strategy at Investor Day
CVS Health Revises Financial Outlook, Showcases Business Strength, and Unveils Bold Strategy to Redefine Health Care at Investor Day CVS Health® will launch its strategy to deliver best-in-class execution, transform…
BioNTech & BMS Report Promising Phase 2 Results for Pumitamig in Advanced Triple-Negative Breast Cancer
BioNTech and Bristol Myers Squibb Unveil Promising Phase 2 Global Data for Pumitamig—First PD-L1xVEGF-A Bispecific Antibody—Demonstrating Encouraging Efficacy in Advanced Triple-Negative Breast Cancer BioNTech SE and Bristol Myers Squibb Company today announced the…


