
Innovate 32 Teams Up with Trivette Osborne & Associates to Grow Tennessee Dental Presence
Innovate 32 Partners with Trivette Osborne & Associates, Expanding its Dental Services Presence in Tennessee Innovate 32 a rapidly expanding, partner-driven dental services organization, announced its partnership with Trivette Osborne…

NextPoint Therapeutics Announces Clinical Debut of Novel NPX372 for Solid Tumor Treatment
NextPoint Therapeutics Announces Clinical Entry of NPX372, a First-in-Class B7-H7–Targeted T Cell Engager to Treat Solid Tumors NextPoint Therapeutics, a clinical-stage biotechnology company developing a new world of precision therapeutics…

Nuvation Bio Launches Global Phase 3 SIGMA Trial of Safusidenib for IDH1-Mutant Glioma
Nuvation Bio Announces Pivotal Global Phase 3 SIGMA Trial (G203) for Safusidenib in IDH1-Mutant Glioma Nuvation Bio Inc. a global oncology company focused on tackling some of the toughest challenges in…

GE HealthCare Unveils ReadyFix Solution to Boost Efficiency and Care Delivery
GE HealthCare launches ReadyFix fleet management solution to help enhance operational efficiency and reliable patient care GE HealthCare today announced the United States launch of ReadyFix™, a remote fleet management…

bioAffinity Technologies Names Leading Pulmonary and Lung Cancer Experts to Advisory Board
bioAffinity Technologies Appoints Nationally Recognized Pulmonary and Lung Cancer Authorities to its Medical and Scientific Advisory Board bioAffinity Technologies, Inc. a biotechnology company focused on noninvasive diagnostics and early cancer detection,…

Estrella Immunopharma Showcases Updated EB103 Data at 2026 ASTCT® & CIBMTR® Tandem Meetings
Estrella Immunopharma Presents Promising Updated Data on EB103 in Oral Presentation at the 2026 Tandem Meetings of ASTCT® & CIBMTR® Estrella Immunopharma, Inc. (Nasdaq: ESLA, ESLAW) a clinical-stage biopharmaceutical company developing…

Bruker Introduces iNTApharma™ Platform to Streamline Nanoparticle Characterization and QC
Bruker Introduces iNTApharma™, a Label‑Free Platform for Nanoparticle Characterization in mRNA Drug and Gene Therapy Development and QC Bruker Corporation today announced the launch of iNTApharma, a label‑free characterization platform that provides single‑particle sensitivity…

VectorY Therapeutics Doses First Participant in Phase 1/2 PIONEER-ALS Trial of VTx-002 for ALS
VectorY Therapeutics Announces First Participant Dosed in Phase 1/2 PIONEER-ALS Clinical Trial of VTx-002 in People with Amyotrophic Lateral Sclerosis (ALS) VectorY Therapeutics, a leader in vectorized antibody therapies for…

Eurofins Viracor BioPharma Broadens Its Bioanalytical Capabilities
Eurofins Viracor BioPharma Expands Bioanalytical Capabilities Eurofins Viracor BioPharma Services, a long‑standing and trusted partner for clinical trial testing solutions with deep expertise in specialty biomarkers and molecular assays, announces…

ZCG-Backed Unimed Completes Acquisition of Regenboog Shipping Pharmacy
ZCG-Backed Unimed Acquires Regenboog Shipping Pharmacy Universal Maritime Solutions a leading global provider of medical and compliance solutions to the maritime industry, today announced the acquisition of Regenboog Shipping Pharmacy, a…

Ethris and DZIF Form Strategic Partnership to Advance Development of mRNA-Based Vaccines
Ethris and German Center for Infection Research (DZIF) Announce Strategic Collaboration to Develop mRNA-Based Vaccines Ethris GmbH, a clinical-stage biotechnology company pioneering next-generation RNA therapeutics and vaccines, and the German Center…
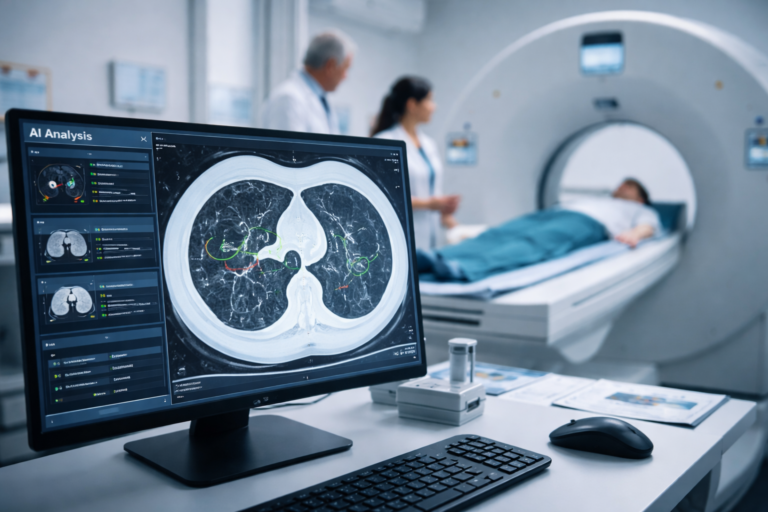
Median Technologies Achieves FDA Clearance for Groundbreaking AI Lung Cancer Screening Technology
Median Technologies Receives FDA 510(k) Clearance for eyonis® LCS, the First AI Tech-Based Detection and Diagnosis Device for Lung Cancer Screening Median Technologies developer of eyonis®, a suite of artificial intelligence…


