
Roche Prasinezumab Shows Potential in Early Parkinson’s Despite Phase IIb Miss
Roche has announced results from the Phase IIb PADOVA study investigating prasinezumab in 586 individuals with early-stage Parkinson’s disease. Participants received treatment for a minimum of 18 months while maintaining…

Merck Reports Phase 3 Results for DOR/ISL HIV Treatment
Merck known as MSD outside of the United States and Canada, today announced topline results from two pivotal Phase 3 trials of the investigational, once-daily, oral, two-drug, single-tablet regimen of…

WHO Academy Launched by Marcron And World Health Organization Leaders
The World Health Organization WHO Academy in Lyon, France, officially opened its doors yesterday with a grand inauguration ceremony. The event was graced by French President Emmanuel Macron, WHO Director-General…
Hansoh Licenses GLP-1 Agonist HS-10535 to MSD Globally
Hansoh Pharma has granted MSD (the tradename of Merck & Co., Inc., Rahway, N.J., USA) an exclusive global license for HS-10535, its investigational oral small molecule GLP-1 receptor agonist. This…
Viatris Publishes Phase 2b CARE Study Data on Cenerimod in Lancet Rheumatology
Viatris announced the publication of Phase 2b CARE study results evaluating the efficacy and safety of cenerimod in adults with moderate-to-severe systemic lupus erythematosus (SLE). The findings, published in Lancet…
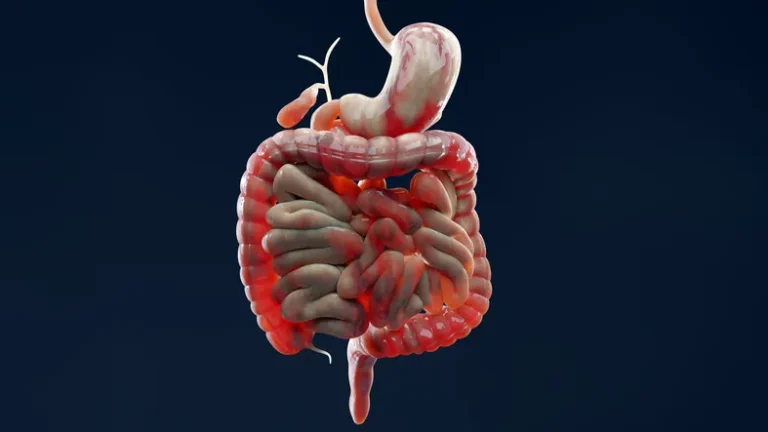
Teva and Sanofi Announce Positive Phase 2b Results for Duvakitug in Ulcerative Colitis and Crohn’s Disease
Teva Pharmaceuticals and Sanofi today announced that the Phase 2b RELIEVE UCCD study of duvakitug (TEV’574/SAR447189), an anti-TL1A monoclonal antibody, met its primary endpoints for the treatment of moderate-to-severe ulcerative…
Aflibercept 8 mg Phase III Study Shows Vision Gains with Extended Treatment Intervals in Retinal Vein Occlusion
Bayer announced positive topline results from the global Phase III QUASAR study, which evaluated the efficacy and safety of aflibercept 8 mg in patients with macular edema following retinal vein…
EYLEA HD 8 mg Phase 3 Trial Shows Improved Vision with Extended Dosing in Macular Edema
Regeneron Pharmaceuticals, Inc announced today that the Phase 3 QUASAR trial investigating EYLEA HD® (aflibercept) Injection 8 mg has successfully met its primary endpoint. The trial evaluated the treatment of…

Lilly and EVA Pharma Announce Regulatory Approval and Launch of Locally Manufactured Insulin in Egypt
The Egyptian Drug Authority has granted regulatory approval for insulin glargine injection manufactured by EVA Pharma in collaboration with Eli Lilly and Company. This approval marks a significant milestone in…

Lilly’s Kisunla (donanemab-azbt) Approved in China for Early Alzheimer’s Treatment
Eli Lilly and Company announced today that China’s National Medical Products Administration (NMPA) has approved Kisunla™ (donanemab-azbt, 350 mg/20 mL every four weeks injection for IV infusion), Lilly’s Alzheimer’s treatment…

Merck Announces FDA BLA Acceptance for Clesrovimab to Protect Infants from RSV
Merck known as MSD outside the United States and Canada, announced today that the U.S. Food and Drug Administration (FDA) has accepted its Biologics License Application (BLA) for clesrovimab (MK-1654),…

Duvakitug Phase 2b Results Highlight Potential in IBD Treatment
Sanofi and Teva Pharmaceuticals, a US affiliate of Teva Pharmaceutical Industries Ltd., have announced promising results from the RELIEVE UCCD phase 2b study, which met its primary endpoints in patients…


