Artesunate AMIVAS, the first fully licensed treatment for severe malaria in Europe, is now available for purchase across Europe and the U.K. through Nordic Prime in Denmark. Approved by the European Commission and the U.K.’s Medicines and Healthcare products Regulatory Agency (MHRA), Artesunate AMIVAS is authorized for use in treating severe malaria in both adults and children, making it the only such licensed product in the region.
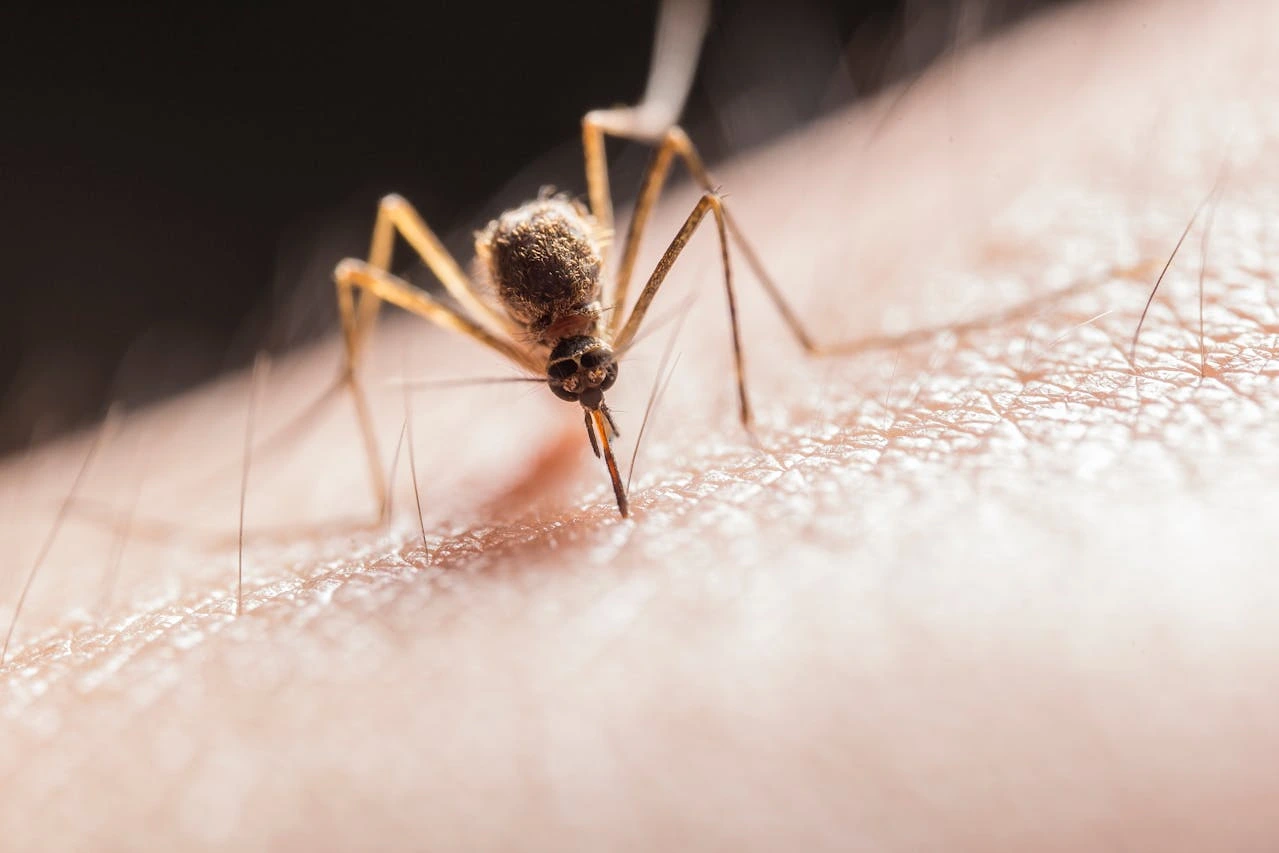
Severe malaria affects around 1,250 people annually in Europe, with most cases arising among military personnel deployed to malaria-endemic regions or travelers returning from such areas. Without treatment, severe malaria has a near 100% mortality rate, with infants, young children, and pregnant women being especially vulnerable. Artesunate AMIVAS, supplied as a 110-milligram powder and solvent for injection, is now distributed across Scandinavia, including Denmark, Finland, Sweden, and Norway, via Nordic Prime.
“The launch of Artesunate AMIVAS across Europe ensures that travelers and military personnel now have access to a licensed, approved treatment that can save lives,” said Laura Walsh, AMIVAS Operations Director. “Its well-established safety and efficacy provide healthcare professionals with confidence when treating severe malaria in patients of any age.”
Sean Power, Director of AMIVAS Ireland, added, “Bringing Artesunate AMIVAS to Europe following its successful U.S. launch represents a significant step towards fulfilling our mission to provide life-saving, fully licensed treatments to those in need. This launch underscores our commitment to extending and improving lives.”
The U.S. FDA approved Artesunate for Injection™ in May 2020 for treating severe malaria in adults and children, with AMIVAS holding the license. The European Commission granted approval for Artesunate AMIVAS in late 2021, followed by the MHRA in April 2022.
About Severe Malaria
Severe malaria, caused by Plasmodium parasites transmitted by mosquitoes, remains a major health threat globally, claiming over 400,000 lives annually, particularly in sub-Saharan Africa. In Europe, although most cases are linked to international travel, climate changes could increase the risk of locally transmitted malaria. The disease can escalate quickly, with untreated cases having a near 100% fatality rate. Vulnerable groups include children under five, pregnant women, and individuals with low immunity.
Artesunate has demonstrated significant benefits, especially for patients with high parasitemia, by reducing the risk of death compared to quinine by up to 34.7%. Complications from severe malaria can include severe anemia, coma (cerebral malaria), respiratory distress, hypoglycemia, and acute kidney injury.
Travelers can take preventive measures before, during, and after travel to malaria-endemic regions to minimize risk.
About Artesunate AMIVAS
Intravenous artesunate has been the standard of care for severe malaria globally for over two decades. Artesunate AMIVAS is approved for the initial treatment of severe malaria in adults and children by the U.S. FDA, European Medicines Agency (EMA), and MHRA. The product, composed of sterile powder for injection, is easily prepared and can be stored at room temperature.
Artemisinins, the active ingredients in Artesunate AMIVAS, are among the fastest-acting anti-malarial compounds and are administered intravenously when formulated for injection.
About AMIVAS
AMIVAS, a biopharmaceutical company based in Nassau, Delaware, was founded to address the need for a U.S.- and European-based manufacturer of regulated artesunate following the discontinuation of quinidine gluconate in 2019. The company secured FDA approval for Artesunate for Injection in 2020 and is dedicated to combating infectious diseases worldwide, advancing scientific breakthroughs to redefine critical care medicines.





