Jazz Pharma Gets EU Nod for Ziihera in Advanced HER2+ Biliary Cancer
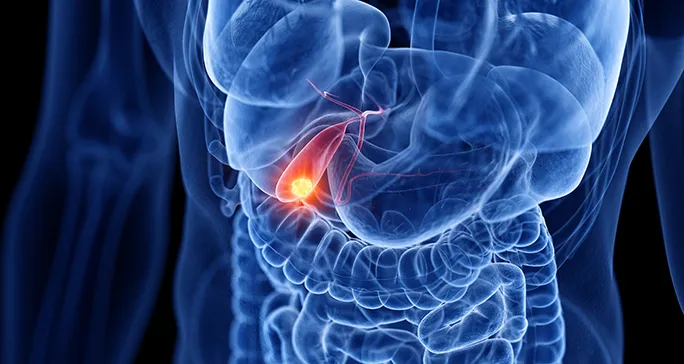
Jazz Pharmaceuticals Secures Conditional EU Approval for Ziihera® (zanidatamab) to Treat Advanced HER2-Positive Biliary Tract Cancer Jazz Pharmaceuticals plc (Nasdaq: JAZZ) has announced that the European Commission (EC) has granted conditional marketing authorization for Ziihera® (zanidatamab), a dual HER2-targeted bispecific…