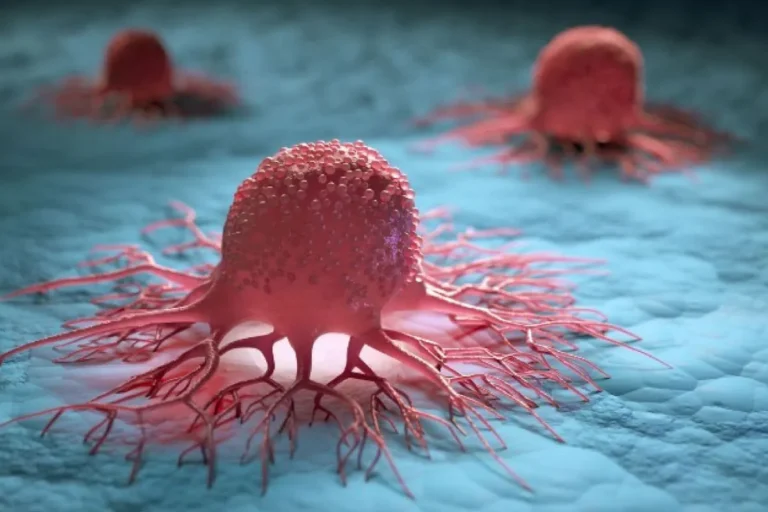
Novocure’s Optune Lua Earns CE Mark Approval for Treating Metastatic Non-Small Cell Lung Cancer
Novocure’s Optune Lua® Earns CE Mark Approval for Treating Metastatic Non-Small Cell Lung Cancer In a significant development for the treatment of advanced lung cancer, Novocure announced that its innovative device, Optune Lua®, has received the Conformité Européenne (CE) Mark,…