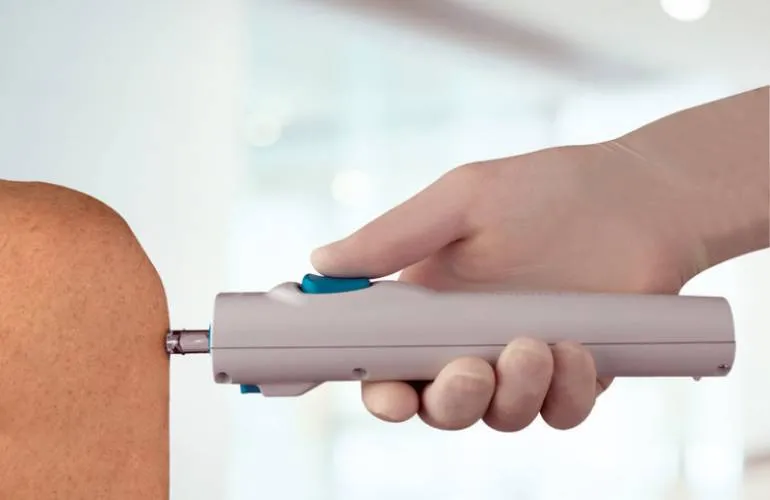
Vaccine Study Shows Fewer Side Effects with PharmaJet Needle-Free Delivery
PharmaJet®, a company committed to advancing the performance, safety, and effectiveness of injectable vaccines and therapies through innovative needle-free injection technology, has announced the publication of a significant new study in the journal Vaccine. This peer-reviewed article details a clinical pilot study evaluating the use of the PharmaJet Tropis® needle-free injection system for administering fractional inactivated poliovirus vaccine (fIPV) doses intradermally (ID) in Cuba’s national immunization program. The findings highlight Tropis as a promising alternative to traditional needle and syringe (N/S) methods, offering notable advantages in safety, ease of use, and patient and healthcare worker satisfaction.
The study, entitled “Tropis needle-free injector for fractional-dose IPV administration: A pilot study for integration into routine immunization services in Cuba”, was designed to explore whether Tropis could be feasibly integrated into Cuba’s existing routine immunization (RI) infrastructure. Cuba currently administers two intradermal doses of fractional IPV to infants at four and eight months of age, in line with global polio eradication strategies. These doses are traditionally delivered using the Mantoux technique with N/S, a method that, while effective, is often technically demanding, uncomfortable, and prone to inconsistencies in execution.
Needle-and-syringe intradermal administration requires specialized training, and improper technique may lead to suboptimal delivery, resulting in diminished immunogenicity of the vaccine. Such limitations can present challenges for scalability and consistency, particularly in settings with limited resources or healthcare infrastructure. The Tropis system, by contrast, automates the process of intradermal delivery using a spring-powered, needle-free device. This approach minimizes the risk of user error and removes the psychological barrier associated with needles, especially for children and caregivers.
PharmaJet’s Tropis technology is the first and only needle-free ID vaccine delivery platform to receive prequalification from the World Health Organization (WHO). It has already been deployed in several global health initiatives, playing a crucial role in mass immunization campaigns in countries like Pakistan, Somalia, and Nigeria. In these nations, Tropis has been instrumental in the administration of polio vaccines to nearly 12 million children, often in hard-to-reach areas and under complex field conditions.
In the Cuban pilot study, researchers from the Instituto Pedro Kourí (IPK) collaborated closely with the WHO to assess the real-world applicability of the Tropis system. A total of 6,704 children were vaccinated using either the Tropis device or traditional N/S methods. The study evaluated three primary parameters: the safety of the injection method, the immunogenic response (as measured by neutralizing antibodies), and the overall acceptability of the technology among healthcare providers and caregivers.
Surveys were distributed to a total of 5,260 parents or guardians and 66 nurses involved in administering the vaccines. These surveys aimed to capture user and provider experiences, measuring satisfaction with the process, perceptions of pain and comfort, and logistical ease. Additionally, blood samples were collected from vaccinated children to assess immunogenicity and confirm that antibody responses were comparable between the two delivery methods.
Results and Key Findings
The outcomes of the study strongly favored the use of the Tropis system over N/S techniques. Adverse events (AEs) associated with vaccination were significantly lower in the Tropis group (6%) compared to the N/S group (13%), with the difference achieving statistical significance (p=0.028). This reduction in side effects is attributed to the precision and consistency of the needle-free delivery mechanism, which avoids the tissue damage or misadministration sometimes seen with manual intradermal injections.
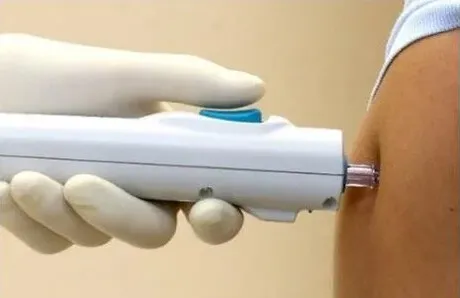
Perhaps even more compelling were the survey results assessing satisfaction among caregivers and healthcare professionals. An overwhelming 98% of parents preferred the Tropis system over N/S, citing reduced crying, shorter procedure times, and increased comfort for their children. Likewise, 98.5% of nurses expressed a preference for Tropis, noting that the system was easier to use, required less training, and eliminated the need for managing and disposing of sharp medical waste.
Importantly, the immunogenicity results showed that the Tropis and N/S groups had no statistically significant differences in seroprevalence. This confirmed that despite the different modes of administration, both delivery methods elicited comparable levels of protective antibodies against poliovirus. In other words, Tropis maintained the efficacy of the fIPV vaccine while providing additional operational and experiential benefits.
A Platform for Innovation in Global Health
The successful implementation of this pilot study in Cuba represents more than a technological upgrade—it exemplifies how innovative health solutions can be seamlessly integrated into existing public health infrastructure. Cuba, with its robust national immunization program and history of effective disease control, has long served as a model for public health innovation. Its collaboration with the WHO, spanning over two decades, has been instrumental in developing, testing, and scaling up strategies aimed at eradicating vaccine-preventable diseases.
This research initiative builds on that legacy, offering new data and insights that could be critical for achieving the endgame goals of the Global Polio Eradication Initiative (GPEI). As the world nears the final stages of eradicating poliovirus, the need for safe, scalable, and acceptable vaccine delivery technologies becomes even more urgent. The success of Tropis in the Cuban context could pave the way for broader adoption in other low- and middle-income countries, particularly in areas where healthcare resources are limited or where training large numbers of providers in complex injection techniques is not feasible.
“Tropis has demonstrated that it is a viable, effective, and more acceptable alternative to the traditional Mantoux technique,” said Paul LaBarre, Vice President of Global Business Development at PharmaJet. “These results are highly encouraging for national immunization programs looking to optimize intradermal delivery. The strong performance of Tropis ID delivery in this study aligns with what we have seen in WHO-sponsored polio campaigns across Pakistan, Nigeria, and Somalia.”
Future Outlook and Global Impact
Looking ahead, the integration of Tropis into routine immunization programs could have a profound impact on global health, particularly as the world seeks to transition from oral polio vaccine (OPV) to IPV-only strategies. The use of fractional doses of IPV administered intradermally is both cost-effective and efficient, but its global uptake has been limited by the challenges of manual ID delivery. Tropis directly addresses those barriers, making it possible to expand fIPV coverage without compromising safety or effectiveness.
Beyond polio, the implications of this technology extend to other diseases as well. Intradermal delivery has been explored for vaccines targeting influenza, rabies, hepatitis B, and even COVID-19. With WHO prequalification already in place, PharmaJet’s Tropis platform could serve as a springboard for broader applications across global immunization campaigns.
In summary, the Cuban pilot study offers robust evidence that needle-free delivery systems like Tropis can transform how life-saving vaccines are administered, especially in underserved and resource-constrained settings. By enhancing safety, reducing discomfort, and improving user satisfaction, Tropis stands to play a pivotal role in shaping the future of vaccine delivery—not just for polio, but across the full spectrum of preventable diseases.





